Egnyte for Life Sciences
Streamline eTMF Management
Inspection-ready eTMF for clinical trials.
Get Life Sciences Demo 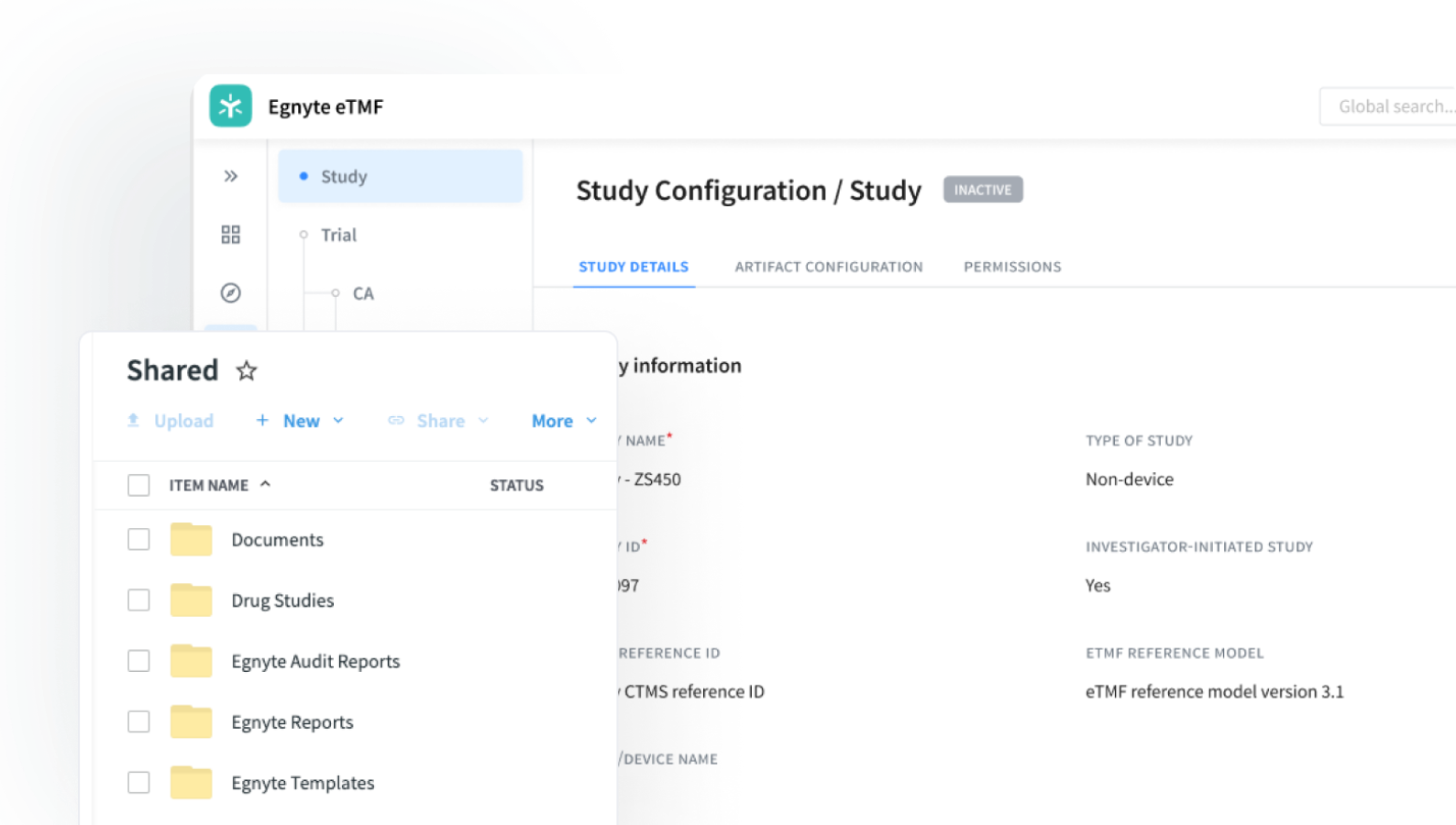
eTMF Made Easy for Biotechs
Assemble all critical trial documents in a cloud-based document management system that grants visibility into trial completeness, quality, and timelines, with built-in audit-readiness for regulatory review. Reduce the time spent on administration of site documentation, searching for and organizing documents, and submission preparation.
Rapid Deployment
Minimize time-to-value with quick and easy set up
Inspection Ready
Ensure all documents are present and accurate with QC reviews and dashboards
Unified Clinical Ops
Leverage the full power of Egnyte’s GxP platform
The Egnyte Solution
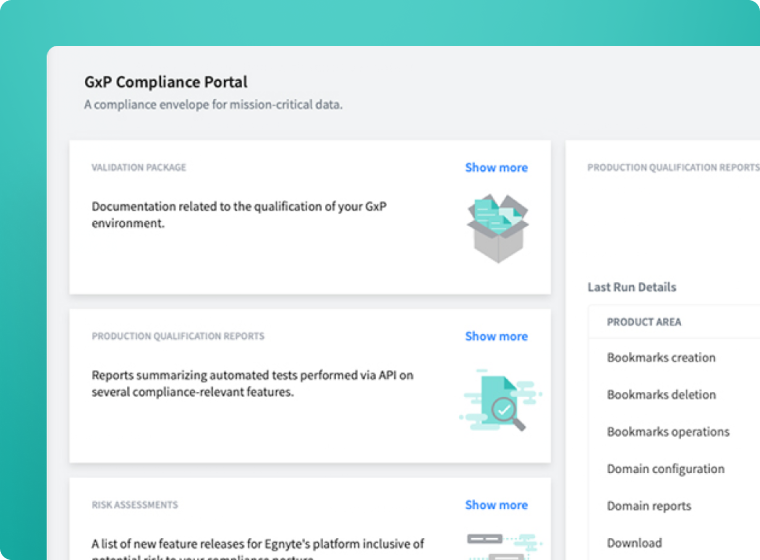
Maintain Compliance
- Stay inspection-ready with TMF completeness metrics
- Support inspections with read-only inspector access
- Manage all TMF documents in a GxP-compliant eTMF system
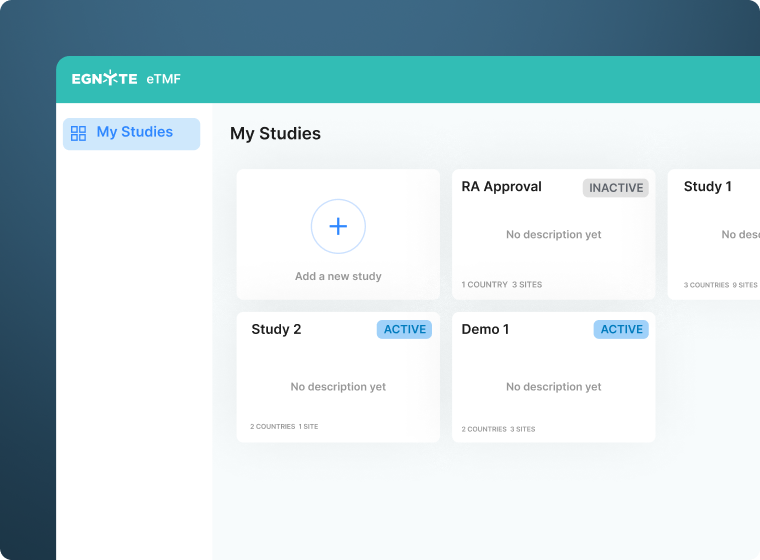
Consolidate Clinical Trial Data
- Collect data across study sites and from sources such an eISF or CTMS
- Centralize data from ongoing and completed studies
- Maintain study data in a secure centralized repository
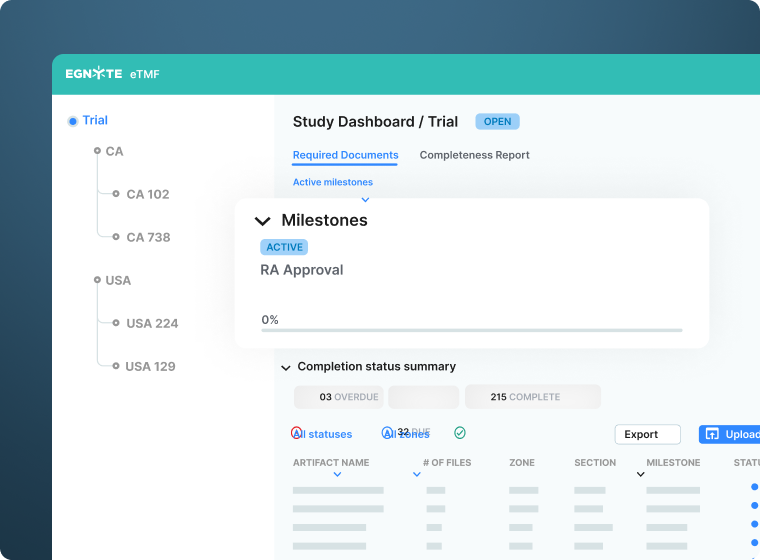
Leverage Actionable Insights
- Monitor study completeness, timeliness and quality in real-time using the study dashboard
- Conduct quality assessments throughout each study
- Export inspection-ready inventory and completeness reports
The Power of Many Solutions In One Turnkey Platform
Egnyte’s modular offerings grow with your company through all development and approval phases - from startup to scale up.
GxP
- GxP Compliance Portal
- Rapid Validation
- Audit Trails
- Multi-Step Workflows
- Data Lifecycle Management
eTMF
Available on Egnyte GxP Platform
- CDISC TMF Reference Model
- Unified Dashboard
- Milestone Management and Reporting
- Multi-version Upload
Over 600 Life Sciences Customers Trust Egnyte
See Egnyte’s eTMF in Action
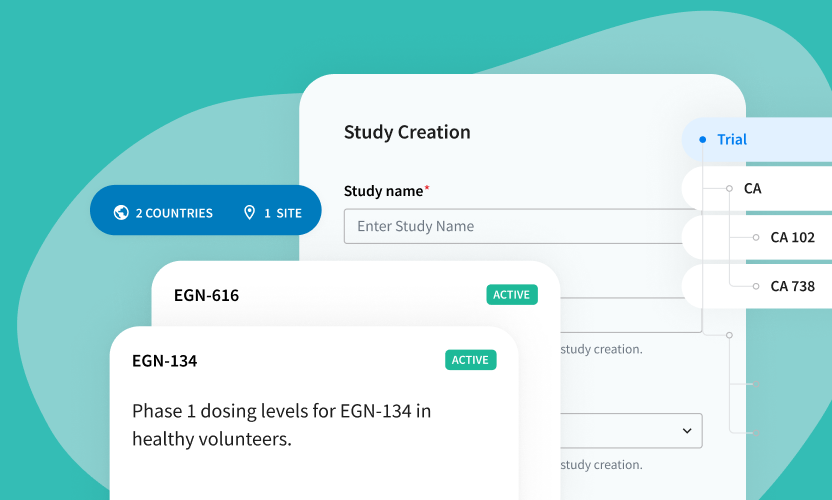
See How Easy It Is to Manage a Study TMF on the Egnyte Platform
Manage your mission-critical regulatory documents to mitigate risk on an industry leading secure & compliant platform.

